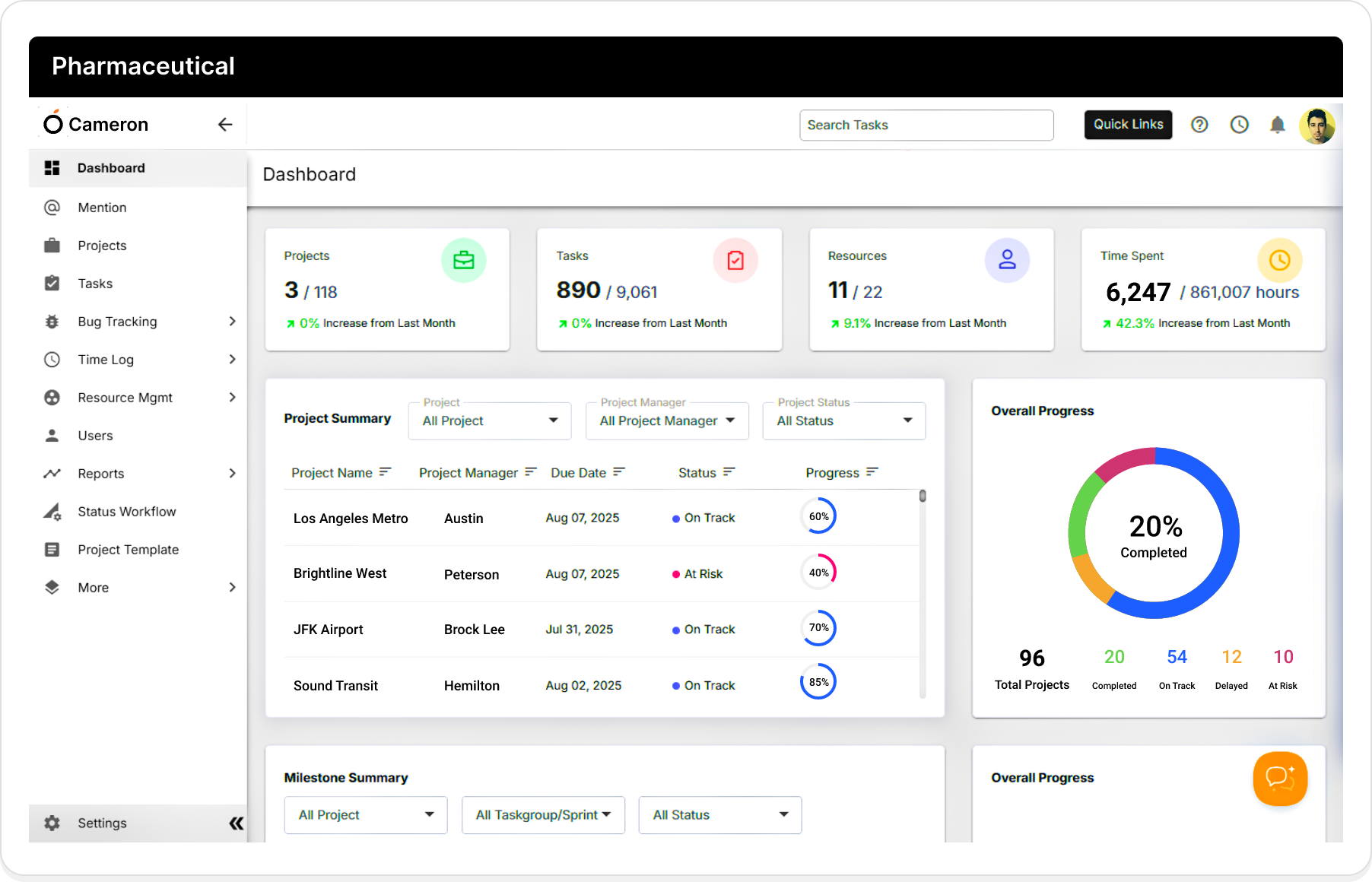
Why Pharma Companies Choose Orangescrum
Streamline R&D, compliance, and collaboration across global teams.
Faster Time-to-Market
Regulatory Compliance Made Simple
Seamless Cross-Functional Collaboration
Resource Optimization
Templates
Designed to meet your unique project management needs, our templates streamline R&D, clinical trials, and compliance processes.
Research Tracking
Monitor research activities, track milestones, and manage experiment data to ensure timely progress.
Team Collaboration
Facilitate communication and coordination among researchers, scientists, and project managers for efficient.
Workflow Automation
Automate routine tasks and approvals to accelerate R&D processes and reduce manual effort.
Questions? We’re Here to Help
Ask us anything about Orangescrum: features, pricing, integrations, or self-hosted setup.
Contact Customer Support
see why teams love working with us.
Features Designed for Pharma Workflows
Manage complex projects, meet strict compliance needs, and drive innovation, without the chaos.
Orangescrum helped broad customers to build their successful workflow!
"Since switching to Orangescrum, our remote teams collaborate better than ever. The real-time updates are a game-changer. We’ve seen a 30% drop in miscommunication."
Emily Rodriguez
Marketing Head
"My team (Hailstorm-Development) and I LOVE Orangescrum! We are a flextime remote business solution specialist agency, and this tool has enabled us to actually create this company. Without you all, we wouldn't even exist!"
Hayley Turner
Founder & CEO, United States, Michigan
"Orangescrum simplifies the process of project management for our organization with its power collaboration tools and provides seamless support and on-boarding. We couldn't be happier with Orangescrum!"
Jamie Smith
Director of Marketing Automation, SFCG, Texas
"We finally have a single place to track tasks, deadlines, and team progress. Orangescrum makes project tracking effortless. Our weekly standups are now more focused and data-driven."
Sneha Nair
Operations Lead
"I work with Freelancers to get the CAD jobs done. Orangescrum provided my team with a way to track and bill their time directly on the project they are working on. This saved me a lot of administrative work."
Brent Kerr
CEO, Kewico GmbH
"We love the customizable workflows in Orangescrum. It adapts perfectly to our internal processes. It feels like the tool was built for us"
Aliya Nurzhanova
Team Lead
"I was very impressed with the ease of use of its interface and all its features to manage projects. It is a platform that can be customized to our needs. Migrating my projects to Orangescrum was super easy."
Clotilde Jolimaitre Rodriguez
Digital Project Manage, Imagevo France
"The most beautiful thing about Orangescrum is its ease in its approach which makes it a lot simpler to use. Orangescrum makes a complicated project way easier to run within my team."
Kuda Msipa
CEO Cutmec Group, Bristol, United Kingdom
"Our major chellenge was to manage multiple Projects/multiple clients at the same time. So we needed something more than excel sheets to manage the development velocity and make things automated."
Shan Sashidharan
Director Of Technology At Techuva Solutions
"Orangescrum helped us streamline project workflows across departments. Task clarity and accountability have improved significantly. It’s now easier to meet deadlines without chaos."
David Miller
Project Manager
Blogs
Stay ahead with the Latest Insights

Stay Tuned for Advanced Reports in Orangescrum

Robotic Process Automation (RPA) in Project Workflow Optimization

Voice-Activated Project Management: The Next Frontier in PMS

AI is the New Project Manager: Hype or Reality?
Success Stories
Explore how they're turning challenges into lasting growth using smarter tools.